6° Clinical case: Facial onset sensory and motor neuronopathy
6° Clinical case: Facial onset sensory and motor neuronopathy
Abstract: This section of Masticationpedia introduces two clinical cases to illustrate the complexities and challenges of differential diagnosis between Orofacial Disorders and more serious organic pathologies. The difficulty in diagnosis often extends beyond clinical skills, stemming from cognitive biases tied to dogmatic approaches and pre-established protocols such as the RDC/TMD, P-values, false positives/negatives, and specialized contexts. The section emphasizes the need for clinicians to critically reflect on these limitations and warns against over-reliance on automated diagnostic models without maintaining a nuanced understanding of patient-specific details.
A clinical case involving a 40-year-old female patient, pseudonymously named ‘Flora,’ who suffered from weight loss, facial tingling, and gum recession after maxillofacial surgery, highlights the complexity of such diagnostic processes. Despite several misdiagnoses, electrophysiological tests, including the jaw jerk and mechanical silent period, led to the discovery of severe trigeminal nerve damage, contradicting the previous diagnosis of a dental issue. The case underscores the significance of context in diagnosis, showing how symptoms might overlap with conditions like Temporomandibular Disorders (TMDs) or Facial Onset Sensory and Motor Neuronopathy (FOSMN), and how cognitive neural network (CNN) models can aid in systematically navigating complex clinical data.
Through a step-by-step investigation, the clinical team bypassed conventional protocols and applied an advanced cognitive diagnostic model to detect FOSMN. Neurological assessments, including trigeminal motor evoked potentials and reflex studies, confirmed the diagnosis. The analysis of the patient’s symptoms and test results, compared with literature, revealed the progression of neuropathy from larger to smaller myelinated fibers, highlighting the critical role of advanced neurophysiological diagnostics in early detection.
The abstract concludes by posing a critical question regarding the pathogenesis of the disorder in the patient’s trigeminal V3 area, suggesting that iatrogenic causes may not fully explain the progression of the disease. The case challenges clinicians to consider multifaceted diagnostic approaches and to remain open to evolving scientific paradigms when dealing with complex neurological and orofacial conditions.
Introduction
In this section of Masticationpedia 'Are we sure to know everything?' we present two emblematic clinical cases that demonstrate the complexity and contextually the difficulty in making a differential diagnosis between Orofacial disorders and serious organic pathologies. These diagnostic difficulties and limits do not only concern the operator's clinical ability, rather the operator's forma mentis too concentrated on pre-established axioms and dogmas. We have already mentioned the ambiguity and vagueness of verbal language logic but we should also be self-critical about established dogmas such as RDC/TMD protocols, P-value,[1] false positives,[2] false negatives and fallacies dictated by specialist contexts etc., so that our infinite clinical intuitive power is amplified and not dampened by easy access to automated machine learning models. This is so true that in an article by Naglaa El-Wakeel,[3] conducted on questionnaires on 151 dentistry professors from Egyptian government and private universities, it emerges that the percentage of diagnostic errors has been estimated to be < 20% and 20-40% of over 90% of the participants. The most commonly misdiagnosed conditions were oral mucosal lesions (83.4%), followed by temporomandibular and periodontal joint conditions (58.9%). The conclusion was that the main causes of this problem are the dental education system and the lack of adequate training. Just a recent report by the National Academies of Science, Engineering and Medicine highlighted a number of shortcomings, particularly in the training of TMDs in dental schools in the United States of America at both the pre-doctoral and post-doctoral (dental) levels, as well as the need to address historical inconsistencies in both diagnosis and treatment. Recently, the American Dental Association recognized orofacial pain as a specialty, which should increase the level and availability of expertise in treating these problems. The article concludes by noting that based on the best current evidence. This report is an attempt to alert the profession to stop irreversible and invasive therapies for the vast majority of TMDs and to recognize that most of these disorders are amenable to conservative and reversible interventions.[4]
Clinical analysis
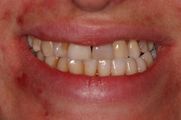
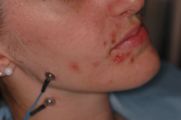
Patient who came to our attention from the Gastroenterology department due to a state of organic food wasting that was difficult to explain in the gastrointestinal diseases. The young patient (aged 40) to whom we give our usual invented name 'Flora' (name of the goddess of flowers in ancient Rome) had undergone maxillofacial surgery for a unilateral crossbite 5 years before she reached our attention. 'Flora' had never had any sensitivity disorder but only an aesthetic problem in smiling and minor masticatory problems such as to turn to a maxillofacial surgeon. The surgery consisted of a rapid surgical palate expansion but after an unquantified period of time there was a recurrence and at the same time slight forms of facial tingling especially in the upper perioral area together with an unexplained gingival recession of the right maxillary dental arch. A few months later, small skin vesicles began to form in her right perioral area, interpreted at the time as a vasculitis manifestation. (Figure 1) Our Flora obviously worried about these consequences relied on dental care both for gum recessions but also for psychological support given the relapses resolved palliatively with a biteplane with the intention of managing night-time stress. Over a period of another 10 months, the patient worried about the conspicuous worsening of her psychophysical conditions and excessive weight loss, decided to refer to a gastroenteric expert who excluded any gastrointestinal pathological form or attributable to malabsorption. (Figure 2) The gastroenterologist colleague had the intuition that perhaps the clinical manifestations of the patient Flora could be attributable to a masticatory difficulty and reported it to our 'Neurognathology' Centre.
Our first approach, for those who have already followed the Masticationpedia diagnostic process, consisted of a quick classic gnathological analysis to which we gave a minimum specific weight given, clearly, the neuropsychophysical conditions in which the patient presented and therefore we bypassed the analysis of assertions in the dental context to immediately perform trigeminal electrophysiological tests.
At the first approach by performing the jaw jerk we realized the seriousness of the clinical situation. The absence of the reflex on the right masseter concomitant with the recession of the right maxillary hemiarch and the skin vesicles gave rise to an important doubt: if we had wanted to attribute the locally performed operation on the maxilla to the intervention, we would have had to expect an electrophysiological deficit attributable to the trigeminal area V2 and not V3 because V2 does not affect the motor responses of the jaw jerk. (Figure 3)
For this reason we have deepened the examination also performing the mechanical silent period of the masseters. (figure 4) The result was striking and confirms the organic damage due to the absence of the jaw jerk and contextually the decrease in duration of the silent period. Already these first two tests directed the pre-diagnosis to an organic/structural damage of the trigeminal nervous system, therefore, a further electrophysiological study of the clinical case was excluded and the patient was immediately directed to the neurophysiology departments.
The ' Demarcation' model was also bypassed so as not to waste further time, but we quickly performed an analysis to look for a more specific morbid form to approach the pre-diagnosis of organic damage. For this reason, the model of the Cognitive Neural Network (CNN) detailed in the previous chapters:
(...... pay attention to the initialization term and the order in which the 'queries' follow one another)
Cognitive Neural Network
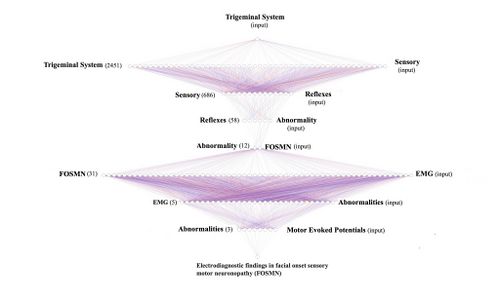
From what emerges from the neurological statements, the 'State' of the Trigeminal Nervous System appears unstructured, highlighting anomalies of the trigeminal reflexes, therefore, the 'Initialization' command is the 'Trigeminal System'to go and test the database (Pubmed).
- 1st loop open: The 'Initialization' command 'Trigeminal System', therefore, is considered as initial input for the Pubmed database which responds with 2,452 clinical/experimental data available to the clinician. The opening of the first true cognitive analysis is elaborated precisely on the analysis of the first result of the 'RNC' corresponding to ' Trigeminal System'. At this stage we realize that the reported datasets include a wide variety of subsets. For this reason we must always remain very generic and insert a corresponding key with an equally broad response that includes one of the signs and/or symptoms found in the anamnesis and clinical analysis. In this case the sensory disturbance reported by the patient will be the second query to be inserted into the network.
- 2st loop open: The 'Sensory' key returns 666 articles on which to do cognitive brainstorming and that is to think about which other element should be inserted in order not to deviate the search from the pre-established set. For example, if in this stage we had entered the term 'jaw jerk' (which has emerged as a decisive test for the diagnosis) the network returns only one article (Differential Diagnosis of Chronic Neuropathic Orofacial Pain: Role of Clinical Neurophysiology). This article concerns a series of tests that can be used for the differential diagnosis in neuropathies but does not help us in researching the type of structural damage the patient is affected by. For this reason it is best to stick to a broad perspective of generic information such as 'Reflexes'.
- 3st loop open: Always bearing in mind that we are still in the starting set (Trigeminal System) the term ' Reflexes' returns 58 scientific articles on which to continue to do cognitive brainstorming (CBing), but how? CBing consists of a dynamic intellectual analysis of the healthcare operator who, knowing the clinical complexity of the clinical case, manages to direct the search for the necessary information by extricating himself from the myriad of database connections that can lead to a dead end, in a sort of node that loses most of the specific information. This CBing is to narrow down to a few articles best related to our clinical case 'Flora'. The generic procedure could be to verify how many articles respond to several terms of clinical question in the same 3rd open loop quantifying their number and value in the context to then better choose the term to insert in the '4st loop closed' which would in fact close the first series of the RCN'. An example could be the following:
- Sensory: searching the text for the term 'Sensory' we will have 10 articles
- Motor: searching the text for the term 'Motor' we will have 6 articles
- Abnormal:searching the text for the term 'Abnormal' we will have only 3 articles on which to dwell to consider the second step of the 'RNC'. The three articles below are very specific in identifying the most suitable path to follow. Details are in the caption. We could have stopped here but the definitive diagnosis is also an important step for the colleagues who will take charge of our patient.
- Therefore the first section of the 'RCN' will conclude with the term 'Abnormality.
- Cognitive Brainstorming su terzo loop open:
Article 3: This article is superimposable on our pre-diagnosis and reports signs and symptoms very similar to our patient, so it is considered as corresponding information and therefore a path to follow. The next step, therefore, will concern the 'Sensory and motor neuronopathy with facial onset' initialed as 'FOSMN'.
- 4st loop closed: The term 'Abnormality'reduces the search to 12 articles which will also be subjected to detailed CBing from which a clinical manifestation very close to the psychophysical state of our patient 'Flora' is extrapolated, a disease coined with the acronym FOSMN which stands for ' Facial onset sensory and motor neuronopathy'.
- 5st loop open: As stated, we are inserting in the database no longer a term but an acronym 'FOSMN' which corresponds to 'Facial onset sensory and motor neuronopathy'. The database returns 31 articles on which to process further requests. Up to now we have used generic terms in order not to lose the connection between nodes but now we need to go into more detail and insert terms that have been highlighted in the clinical and laboratory analysis such as, for example, electromyographic anomalies for which we insert the term 'EMG' .
- 6st loop open: The term 'EMG' drastically reduces the CBing to only 5 items to be carefully weighed to continue with the 'RNC' and since a serious EMG abnormality of amplitude and duration was highlighted in our 'Flora' we insert the term 'Abnormalities' in the database.
- 7st loop open: Of the three articles on the term'Abnormalities' he replies with returning 3 articles and we preferred to insert a more specific and advanced term of scientific studies, the 'motor evoked potentials' since this pathology is a sensory and motor clinical manifestation.
- 8st loop open: By inserting the term 'motor evoked potentials' in the database we have reached a conclusion point the typical 'Loop closed' that returns the article 'Electrodiagnostic findings in facial onset sensory motor neuronopathy (FOSMN)'.
- 9st loop closed: As we have seen, the 'RNC' is a cognitive network model that helps the clinician to unravel the diagnostic complexity by searching, precisely, with a human cognitive dynamic and not machine learning, for a possible overlap of clinical elements as well as decrypting the encrypted signal sent out by the body. As we have verified, in fact, specifically in the cases of Mary Poppins and 'Bruxer'. However, of the 2452 articles of the set identified with the initialization world we arrived with only 8 loops to extract only one article 'Electrodiagnostic findings in facial onset sensory motor neuronopathy (FOSMN)'' on which to do further brainstorming.
Final diagnosis
The patient 'Flora' was, therefore, immediately referred to the trigeminal neurophysiology departments with a pre-diagnosis of 'Electrodiagnostic findings in facial onset sensory motor neuronopathy (FOSMN)' and we report the procedure that confirmed the diagnosis to make it more the diagnostic path that a clinical dentist should follow in such rare but dramatically serious cases is explanatory. In fact, the diagnosis is not linear in the first cases because the symptoms could be superimposed on various physiopathogenetic phenomena such as temporomandibular disorders (TMDs), trigeminal neuralgia (TN), forms of trigeminal neuropathies such as 'isolated sensory trigeminal neuropathy' (TISN ) and that identified in this clinical case 'Facial onset sensory and motor neuronopathy' (FOSMN). This diagnostic process is required because in the case of TISN or FOSMN the prognosis is often poor.
In Prof. Cruccu's Department of Neurology and Clinical Neurophysiology, the patient underwent the following tests:
Clinical and laboratory investigations
Trigeminal and extra-trigeminal sensory function were assessed: touch was studied with a cotton ball, vibration with a tuning fork (128 Hz), and pin-prick sensation with a wooden cocktail stick. Gait impairment and muscle strength were assessed with the Medical Research Council score. We were also asked to report dysautonomic symptoms. The patient underwent laboratory tests, including tests to rule out identifiable causes of trigeminal neuropathy: autoantibody assays to detect connective tissue disease (antinuclear antibodies, anti-double stranded DNA, antinuclear extractable antigens, including anti Sm, anti RNP, anti Scl70 and anti -phospholipids, antineutrophil cytoplasmic antibodies and anti Ro/SSA and anti-La/SSB for Sjögren's disease).
Serum genetic testing for Kennedy disease, cholesterol esters and low serum cholesterol for Tangieri disease, glycosphingolipid accumulation for Fabry disease, and serum angiotensin converting enzyme for neurosarcoidosis. Of course, gadolinium-enhanced magnetic resonance imaging (MRI) of the brain and spinal cord was also performed.
Supraorbital nerve biopsies were performed by an experienced plastic surgeon. Samples fixed with 2% glutaraldehyde in phosphate buffered saline (PBS) at 4°C. Samples were post-fixed in 1% osmium tetroxide in veronal acetate buffer (pH 7.4) for 1 hour at 25°C, stained with uranyl acetate (5 mg/ml) for 1 hour at 25°C C, dehydrated in acetone and incorporated in Epon 812 (EMbed 812, Electron Microscopy Science, Hatfield, PA, USA).
Semithin sections were stained with toluidine blue for light microscopic evaluation. Ultrathin sections from tissue blocks in the correct orientation, post-stained with uranyl acetate and lead hydroxide, were examined with a Morgagni 268D transmission electron microscope (FEI, Hillsboro, OR, USA). Digital images were analyzed with AnalySIS software (SIS) and all myelinated and unmyelinated structures were identified and measured. Fiber densities were calculated and expressed as average number of fibers/mm2.
Trigeminal neurophysiology
Trigeminal motor evoked potentials were tested by transcranial magnetic stimulation,[5] the temporal H reflex, evaluating the Aα fiber (Ia fiber) in the monosynaptic trigeminal reflex,[6] the early components of the blink reflex (R1) after electrical stimulation of the supraorbital nerve, and the masseter inhibitory reflex (SP1) after stimulation of the mental nerve, assessing the fibers .[7] Laser-evoked potentials (LEP) were also recorded to study nociceptors (-LEP) and unmyelinated fiber heat receptors (C-LEP).[8] The neurophysiological tests have adhered to the technical requirements issued by the International Federation of Clinical Neurophysiology.[9][10]
Significant results of the investigations
Motor evoked potentials from transcranial magnetic stimulation and the temporalis muscle H reflex yielded normal results; in contrast, reflex recordings showed severe abnormalities: the first response to become absent bilaterally was the early masseter inhibitory reflex (ES1) after mental nerve stimulation and the early blink reflex (R1). While fiber-mediated LEPs were often abnormal (but less impaired than early trigeminal reflexes), unmyelinated C-fiber-mediated C-LEPs were normal. These patterns of neurophysiological abnormalities generally suggested that the disease progressed from larger to smaller afferent fibers.
The one notable exception was the normal temporal H reflex afferent-mediated by . Both light and electron microscopy showed only Wallerian-like degeneration involving myelinated fibers, more severe for the large and small group , with no inflammatory changes.
Discussion
From the study by Cruccu et al.[11] first of all it is deduced that despite detailed neurophysiological and morphometric investigations, no clinical, neurophysiological or neuropathological differences can be found between TISN and FOSMN, therefore, the two diseases could be pathophysiologically similar neuropathies of the type of dissociated neuropathies that completely spare the unmyelinated fibers as demonstrated by the biopsy exam. Light and electron microscopy in supraorbital nerve biopsy specimens from patients with TISN and those with FOSMN have shown variously severe axonal myelinated fiber loss, as others have reported in these patients.[12][13] We extend these findings by providing quantitative data showing that trigeminal neuropathy affects fibers more severely than fibers. Evidence that nerve fiber damage progresses from the largest to the smallest fiber also comes from neurophysiological findings, which invariably show responses mediated by the impaired fiber even in the early stages of the disease. In contrast, fiber-mediated responses were much less impaired.
According to Cruccu et al.[11] temporal sparing of the H reflex provides evidence that FOSMN primarily affects cell bodies.[13][14] A dissociated neuropathy that progressively affects larger and then smaller myelinated fibers should in theory severely impair a reflex mediated by afferents from muscle spindles. Conversely, it spares the primary afferents from the trigeminal spindles because they travel in the motor rather than the sensory root. Equally important, rather than lying in the sensory ganglion, their cell bodies lie in the midbrain trigeminal nucleus.[15][16] This unique anatomical feature also explains why mandibular tendon snapping (or jaw snapping) is spared in two other trigeminal neuropathies: Sjögren's syndrome and Kennedy's disease.[17][18]
Unlike previous studies that used a hand-held reflex hammer to elicit mandibular tendon snap, we used the temporal H reflex to avoid possible interference from temporomandibular dysfunction or malocclusion, conditions that can induce abnormal or even absent reflex response to the reflex. hand held hammer.[19]
A Hamletic doubt arises:
This datum is of essential importance for the clinical scientific model proposed by the Masticationpedia group, that of 'Probabilistic Indetermination' in organic complex systems, for which laboratory tests in many cases, could highlight anomalies that would otherwise be hidden and which would have been highlighted with long temporal latencies and severe clinical repercussions. In particular, given the physopathogenetic results that emerged from Cruccu's work[11] and specifically in our patient 'Flora', it means that the anomalous results highlighted in our pre-diagnosis period in the patient (jaw jerk and silent period abnormalities) were an abnormal clinical manifestation already present even before the disease had spread further to the smaller caliber myelinated fibers. In a nutshell, the paresthesia reported by the patient was pathognomonic of damage to the myelin fibers after a process of deconstruction of the midbrain nuclei and damage to the fibers.
However, an even more complex doubt remains to be resolved:
If the organic damage concerns the motor nervous structures with a progression from the fibers to the fibers with consequent structural damage of the midbrain motor nuclei, initially sparing the -unmyelinated small caliber fibers and the orthognathic surgery was performed only in the maxilla, how can the initialization be explained of the disease manifesting mainly in the trigmeinal area V3?
(....... are we still sure we know everything?)
- ↑ S Catarzi, D Morrone, D Ambrogetti, P Bravetti, M Rosselli Del Turco, S Ciatto[Errors in mammography. II. False positives] Radiol Med. 1992 Mar;83(3):201-5.
- ↑ D Morrone 1, D Ambrogetti, P Bravetti, S Catarzi, S Ciatto, M Rosselli del Turco[Diagnostic errors in mammography. I. False negative results]. Radiol Med.1991 Sep;82(3):212-7.
- ↑ El-Wakeel N, Ezzeldin N. Diagnostic errors in Dentistry, opinions of egyptian dental teaching staff, a cross-sectional study. BMC Oral Health. 2022 Dec 20;22(1):621. doi: 10.1186/s12903-022-02565-9.PMID: 36539763
- ↑ Gary D Klasser, Elliot Abt, Robert J Weyant, Charles S Greene. Temporomandibular disorders: current status of research, education, policies, and its impact on clinicians in the United States of America. Quintessence. 2023 Apr 11;54(4):328-334.doi: 10.3290/j.qi.b3999673.
- ↑ Cruccu G, Berardelli A, Inghilleri M, Manfredi M. Functional organization of the trigeminal motor system in man. A neurophysiological study. Brain. 1989;112:1333–1350. doi: 10.1093/brain/112.5.1333.
- ↑ Cruccu G, Truini A, Priori A. Excitability of the human trigeminal motoneuronal pool and interactions with other brainstem reflex pathways. J Physiol. 2001;531:559–571. doi: 10.1111/j.1469-7793.2001.0559i.x.
- ↑ Valls-Solé J. Neurophysiological assessment of trigeminal nerve reflexes in disorders of central and peripheral nervous system. Clin Neurophysiol. 2005;116:2255–2265. doi: 10.1016/j.clinph.2005.04.020.
- ↑ Cruccu G, Pennisi E, Truini A, Iannetti GD, Romaniello A, Le Pera D, De Armas L, Leandri M, Manfredi M, Valeriani M. Unmyelinated trigeminal pathways as assessed by laser stimuli in humans. Brain. 2003;126:2246–2256. doi: 10.1093/brain/awg227.
- ↑ Kimura J, editor. Peripheral Nerve Diseases, Handbook of Clinical Neurophysiology.Amsterdam: Elsevier; 2006.
- ↑ Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, Garcia-Larrea L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–1719. doi: 10.1016/j.clinph.2008.03.016.
- ↑ 11.0 11.1 11.2 Cruccu G, Pennisi EM, Antonini G, Biasiotta A, di Stefano G, La Cesa S, Leone C, Raffa S, Sommer C, Truini A.Trigeminal isolated sensory neuropathy (TISN) and FOSMN syndrome: despite a dissimilar disease course do they share common pathophysiological mechanisms? BMC Neurol. 2014 Dec 19;14:248. doi: 10.1186/s12883-014-0248-2.PMID: 25527047
- ↑ Lecky BR, Hughes RA, Murray NM. Trigeminal sensory neuropathy. A study of 22 cases. Brain. 1987;110:1463–1485. doi: 10.1093/brain/110.6.1463.
- ↑ 13.0 13.1 Vucic S, Tian D, Chong PS, Cudkowicz ME, Hedley-Whyte ET, Cros D. Facial onset sensory and motor neuronopathy (FOSMN syndrome): a novel syndrome in neurology. Brain. 2006;129:3384–3390. doi: 10.1093/brain/awl258.
- ↑ Vucic S1, Stein TD, Hedley-Whyte ET, Reddel SR, Tisch S, Kotschet K, Cros D, Kiernan MC. FOSMN syndrome: novel insight into disease pathophysiology. Neurology. 2012;79:73–79. doi: 10.1212/WNL.0b013e31825dce13.
- ↑ Collier TG, Lund JP. The effect of sectioning the trigeminal sensory root on the periodontally-induced jaw-opening reflex. J Dent Res. 1987;66:1533–1537. doi: 10.1177/00220345870660100401.
- ↑ Dessem D, Taylor A. Morphology of jaw-muscle spindle afferents in the rat. J Comp Neurol. 1989;282:389–403. doi: 10.1002/cne.902820306.
- ↑ Valls-Sole J, Graus F, Font J, Pou A, Tolosa ES. Normal proprioceptive trigeminal afferents in patients with Sjögren's syndrome and sensory neuronopathy. Ann Neurol. 1990;28:786–790. doi: 10.1002/ana.410280609.
- ↑ Antonini G, Gragnani F, Romaniello A, Pennisi EM, Morino S, Ceschin V, Santoro L, Cruccu G: Sensory involvement in spinal-bulbar muscular atrophy (Kennedy's disease).Muscle Nerve 2000; 23:252-258.
- ↑ Cruccu G, Iannetti GD, Marx JJ, Thoemke F, Truini A, Fitzek S, Galeotti F, Urban PP, Romaniello A, Stoeter P, Manfredi M, Hopf HC. Brainstem reflex circuits revisited. Brain. 2005;128:386–394. doi: 10.1093/brain/awh366.